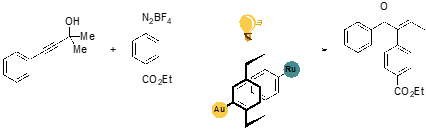Metal-Complexes based on [2,2]Paracyclophan
[2,2]Paracyclophan
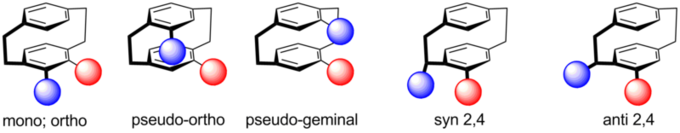
Configurations of paracyclophanes
This subgroup’s task is the synthesis of geometrically fixed, chiral polynuclear organometallic complexes and the investigation of their catalytic and optical properties. We use di- and higher functionalized [2.2]paracyclophanes as well as [2](1,4)benzeno[2](2,5)pyridinophanes with different donor motifs as ligand systems. The rigid structure of the paracyclophane framework ensures a defined structural relation of the complexed metal centers to each other. The obtained complexes are tested in different reactions as potential asymmetric multi-metal catalysts and, in collaboration with other groups, the cooperation of the metal centers and their optical properties are investigated.
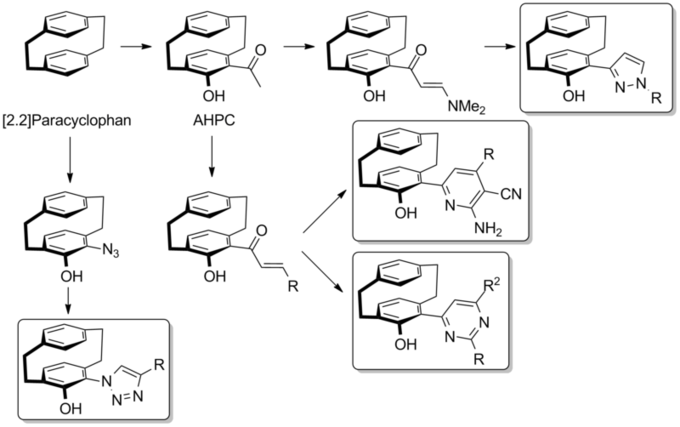
Synthetic routes of different PC derivatives
Starting from [2.2]paracyclophane 4-acetyl-5-hydroxy-[2.2]paracyclophane (AHPC) as well as pyrazole-derivatives were synthesized already. The current research focuses on the synthesis of pyridine-, pyrimidine- and triazole-derivatives.
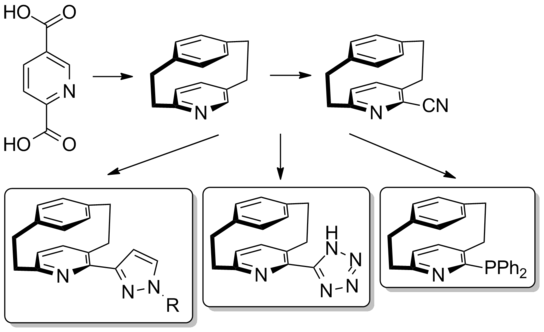
Synthetic routes for the pyridinophan framework
Similar synthetic routes are available for the pyridinophane framework, while this framework already shows chirality in the unsubstituted form.
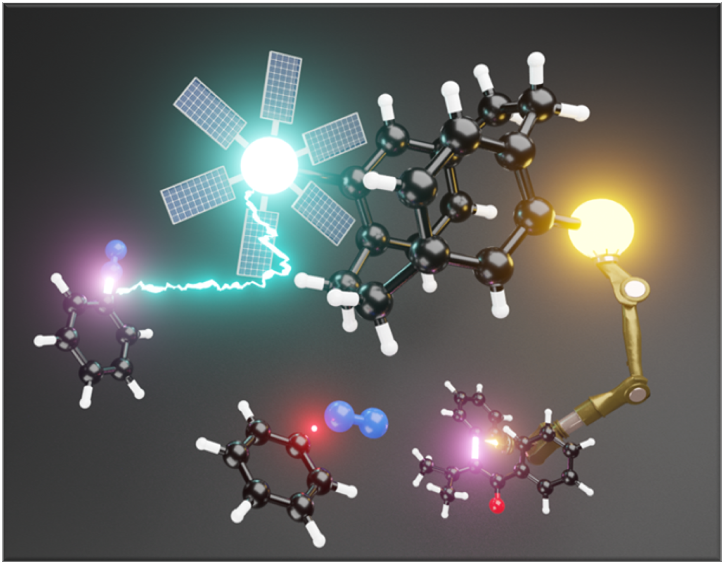
Investigation of cooperative effects in a distance defined Au(I)/Ru(II) heterobimetallic photoredoxcatalyst based on [2.2]paracyclophane isomers
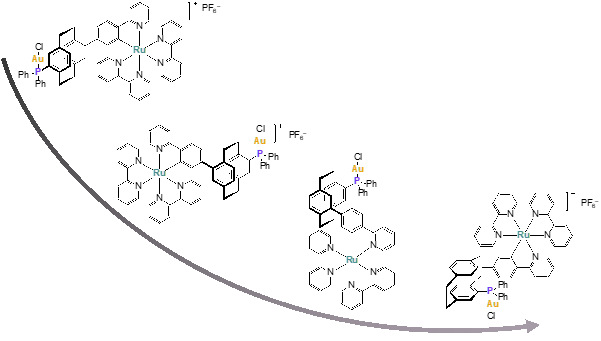
The development of novel molecular architectures to synthesize heterobimetallic catalyst systems for visible-light mediated photoredoxcatalysis has been an outstanding objective. The synthesis, characterization and catalytic application of a set of distance-defined Au(I)/Ru(II) heterobimetallic complexes was developed.[1] The rigid [2.2]paracyclophane serves as a modular backbone and allows to hold two metal centers in a fixed spatial orientation and distance to one another.
The unique “bent and battered” structure of [2.2]paracyclophane features planar chirality, high rigidity and transannular communication[2-5] which makes this scaffold an ideal playground for the synthesis of distance-defined bimetallic complexes.
Disubstituted PCPs with two substituents located on different decks, display four different regioisomers: pseudo-geminal, -ortho, -meta and -para. The distance between the substituents increases in this order. If the substituents are designed to act as selective binding sites, a framework is generated on which cooperative effects which rely on spatial proximity or electronic communication can be studied. The ground state is investigated by UV/Vis spectroscopy and cyclic voltammetry and the excited state is studied by transient absorption spectroscopy in cooperation with the groups of Diller and Riehn at University of Kaiserslautern to elucidate the underlying ultrafast photophysics and -chemistry as well as metal-metal-interactions.
[1] D. M. Knoll, C. Zippel, Z. Hassan, M. Nieger, P. Weis, M. M. Kappes, S. Bräse, Dalton Trans. 2019, 48, 17704-17708.
[2] D. J. Cram, J. M. Cram, Acc. Chem. Res. 2002, 4, 204-213. [3] I. Majerz, T. Dziembowska, J. Phys. Chem. 2016, 120, 8138–8147.
[4] Z. Hassan, E. Spuling, D. M. Knoll, J. Lahann, S. Bräse, Chem. Soc. Rev. 2018, 47, 6947–6963.
[5] Z. Hassan, E. Spuling, D. M. Knoll, S. Bräse, Angew. Chem. Int. Ed. 2020, 59, 2156.