What is TADF and How Does the Molecule Design Work?
Thermally activated delayed fluorescence (TADF) molecules typically display a small energy gap (ΔEST) between the lowest-lying singlet state S1 and lowest-lying triplet state T1. When the lifetime of the T1 excitons is long enough, a process called reverse intersystem crossing (RISC) is thermally activated and the triplet excitons are up-converted into the S1 state. From there, they radiatively relax to the ground state S0. Through the up-conversion process a theoretical internal quantum efficiency (IQE) of 100% can be achieved.
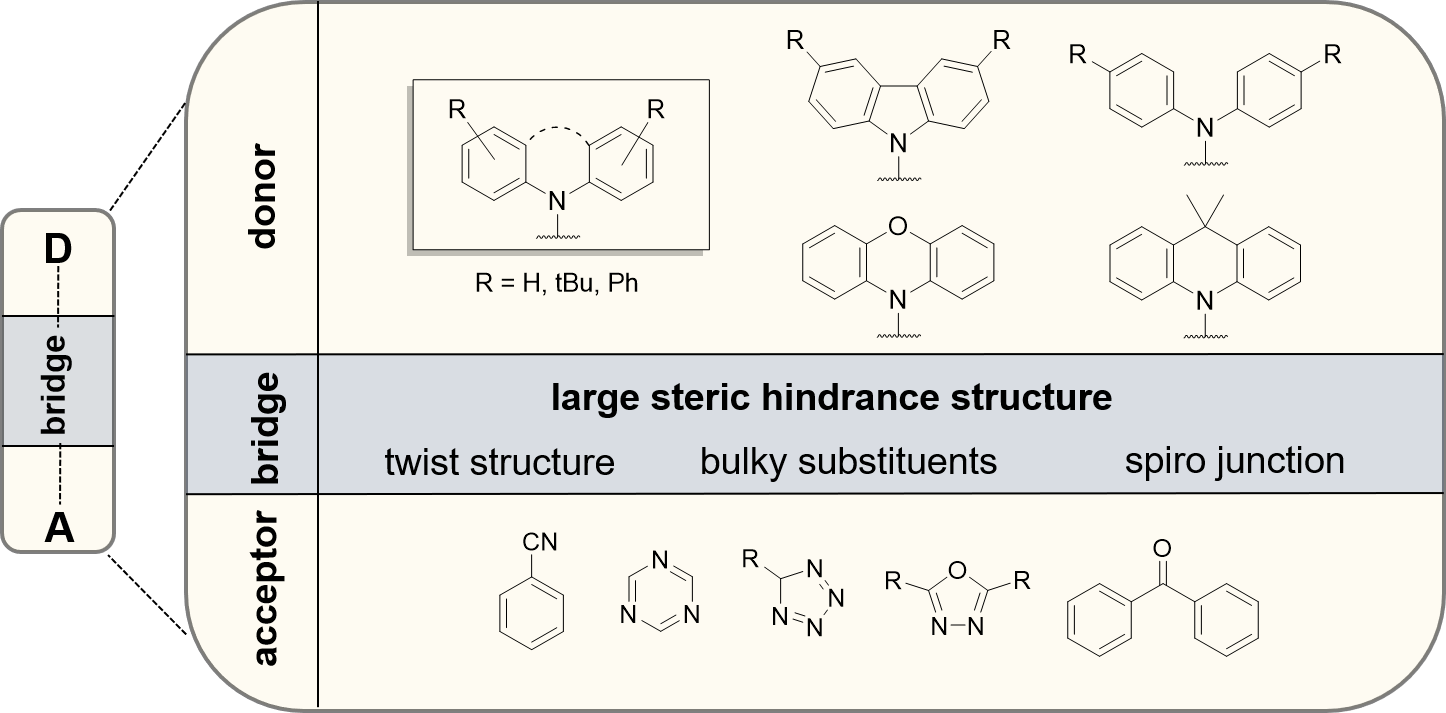
TADF emitters can be strategically modulated using electron-donating and electron-accepting groups. A well-investigated approach is the design of a TADF emitter capable of a twist-induced charge-transfer. Often, a spacing group is interconnected which is capable to vary the dihedral angle between donor and acceptor. Emitters can be designed with the donor directly linked to the acceptor, varying the account of donating/accepting groups, or interconnecting a bridging unit. Arylated N-heterocyclic compounds like carbazoles and acridines as well as triphenylamines are suitable donor moieties. On the other side, widely used acceptor groups include benzonitriles, tetrazoles, triazoles, oxadiazoles, triazines, benzophenones and sulfones.
Figure 1. Donor and acceptor structures used in TADF molecules.
TADF molecules can be applied in organic light emitting diodes (OLEDs) as fluorophores or in cellular imaging as dyes. The TADF team at AK Bräse specializes on the design and synthesis various types of TADF molecules for the application in these fields based on purely organic structures, metal complexes and polymers which will be explained in further detail when you follow the individual tabs.